Evo Devo Universe and Information Philosopher, October 2, 2025
In my
Self-Organization in Complex Systems talk in Siena for Georgi's CCS25 meeting September 4 and in the
September 12 Evo Devo Scholar's Talk, I described
Laplace's intelligent demon and
Seth Lloyd's universe as a computer.
They both view the information in the universe as a
conserved constant.
We also discussed
Lord Kelvin and
Herman Helmholtz's prediction of a universe
heat death.
But complex information structures are not constant and they are certainly not decreasing!
I described my work following the ideas of
Arthur Stanley Eddington and
David Layzer.
I showed how the expansion of the universe has been avoiding the second law of thermodynamics from the first moments of time, creating information structures from elementary particles to stars like our Sun and planet Earth with its
biosphere.
I adapted the diagrams in Layzer’s 1989 book
Cosmogenesis to create the
Eddington/Layzer picture of the growth of information (and of entropy) in the universe.
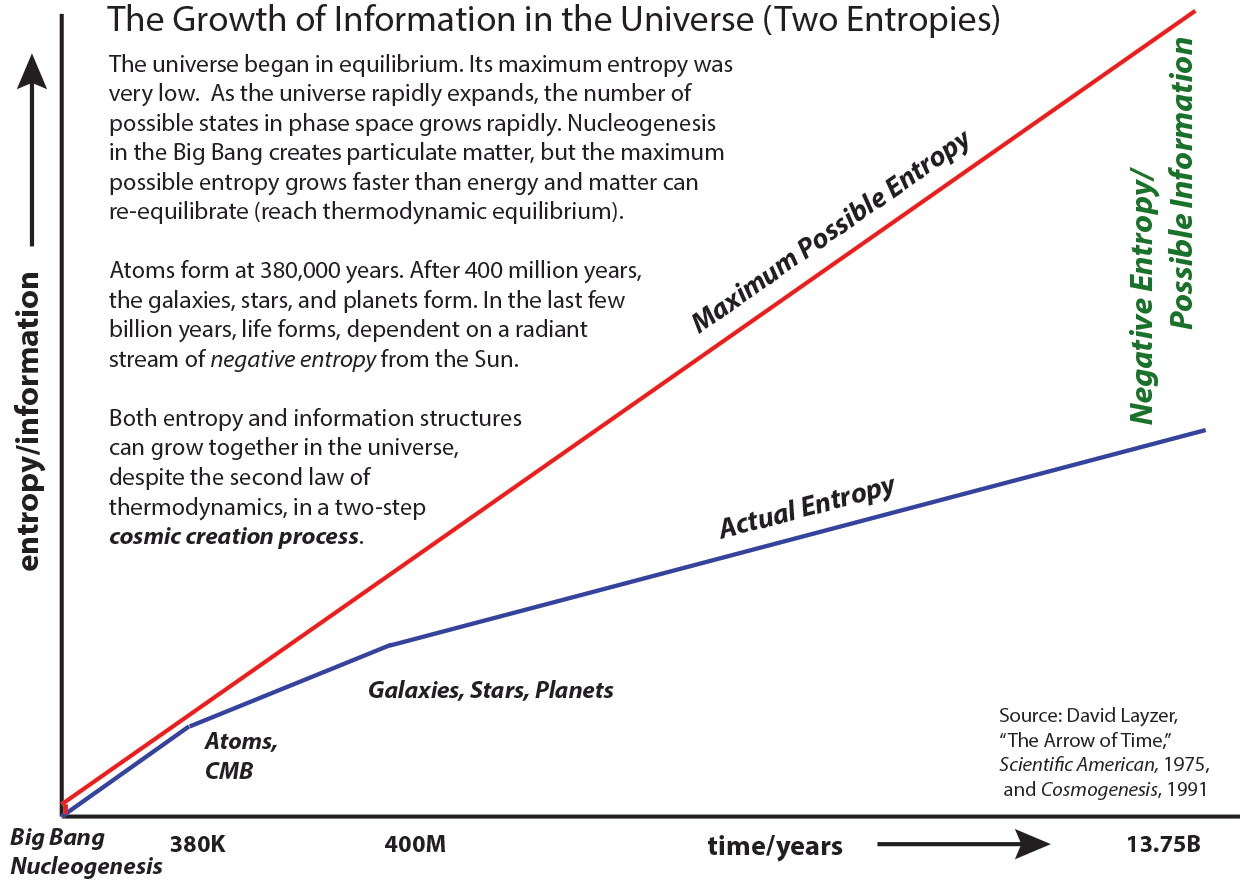
Between the
actual entropy and the
maximum possible entropy you can see there's plenty of room for galaxies, stars, and planets to form.
The expansion of space creates new
possible locations in phase-space, producing pockets of negative entropy. When an
actual information structure forms
locally, it will not be stable unless it radiates away positive entropy to satisfy the second law
globally.
I call these two steps, first
possibilities, then one
actuality, the
cosmic creation process.
These
two steps or
two stages are first
indeterministic (random) possibilities, second an
adequately determined (not
pre-determined!) choice or selection.
This is exactly how
Claude Shannon's theory of the communication of information works, showing the intimate connection between negative entropy and information!
Information philosophy proposes four such processes, all driven by random possibilities.
-
For Shannon, there must be multiple possible messages. If there is only one possible message, no information is communicated.
- For the universe, without the expansion creating new phase-space possibilities, the universe would be closed and suffer a "heat death"!
- Ernst Mayr called Darwinian evolution a two-step process. Without chance variations, there would be no new species.
- For the two-stage model of human free will, without the mind producing random new thoughts, there would be no free actions.
The universe began with primeval quarks, gluons, electrons, and photons. In the first few minutes after the origin, the
cosmic creation process produced the earliest information structures, protons and neutrons. 380,000 years later, the ionized plasma cooled to the surface temperature of the Sun and allowed those protons and electrons to form atoms, making the universe transparent. That allowed us today to see back in time to the cosmic microwave background.
Galaxies, stars, and planets began to form about 400 million years after the origin.
The Sun, a population I star, formed only about 4.5 billion years ago, along with its planets, and
life emerged rather quickly about a half-billion years later.
We discussed how
Erwin Schrödinger famously argued that
life “feeds on negative entropy.”
Schrödinger’s source for negative entropy was our Sun. With the bright Sun as a heat source and the dark night sky as a heat sink, the Earth is a thermodynamic engine.
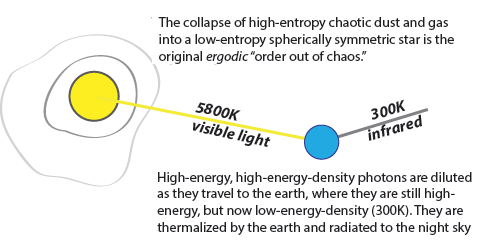
But Schrödinger
didn't know how the Sun (and all the stars) came to be such a source of negative entropy. Following Eddington and Layzer, I have explained that with the
cosmic creation process.
Paul Davies and David Layzer
Davies' first book
The Physics of Time Asymmetry, in 1974, explored the "
arrow of time." In the 1977 second edition Davies cited
David Layzer's 1975 article
The Arrow of Time in
Scientific American. That article and Layzer's 1989 book
Cosmogenesis: The Growth of Order in the Universe are critical for understanding Davies' latest thinking on the origin of life.
Twenty years later, Davies and two colleagues edited the 1994 volume
Physical Origins of Time Asymmetry.
Davies contributed the article, "Stirring up Trouble." Without mentioning
David Layzer's 1975 "Arrow of Time" article or Layzer's 1989 book on the
Growth of Order, Davies coined the term "entropy gap" to describe Layzer's insight that the maximum possible entropy goes up faster than the actual entropy.
Does this transition from equilibrium to disequilibrium not constitute a violation
of the second law of thermodynamics? No. What has happened is depicted in Fig.
3. At some time around one second, the material content of the universe was in
a state of equilibrium, having the maximum possible entropy for the constraints
at that time. As the universe expanded, however, the maximum possible entropy
rose. The actual entropy also rose, but less fast. In particular, the relaxation time
for nuclear processes to allow the cosmological material to keep pace with the
changing constraints (due to the expansion) was much longer than the expansion
time, so the material began to lag further and further behind equilibrium conditions
( equilibrium meaning in the nuclear case that this material is in the form of the, most
stable element - iron). Hence an 'entropy gap' opened up. The continuing expansion
of the universe serves to try and widen that gap slightly (though now through other
processes than nucleosynthesis), while physical processes such as starlight production
serves to try and narrow it.
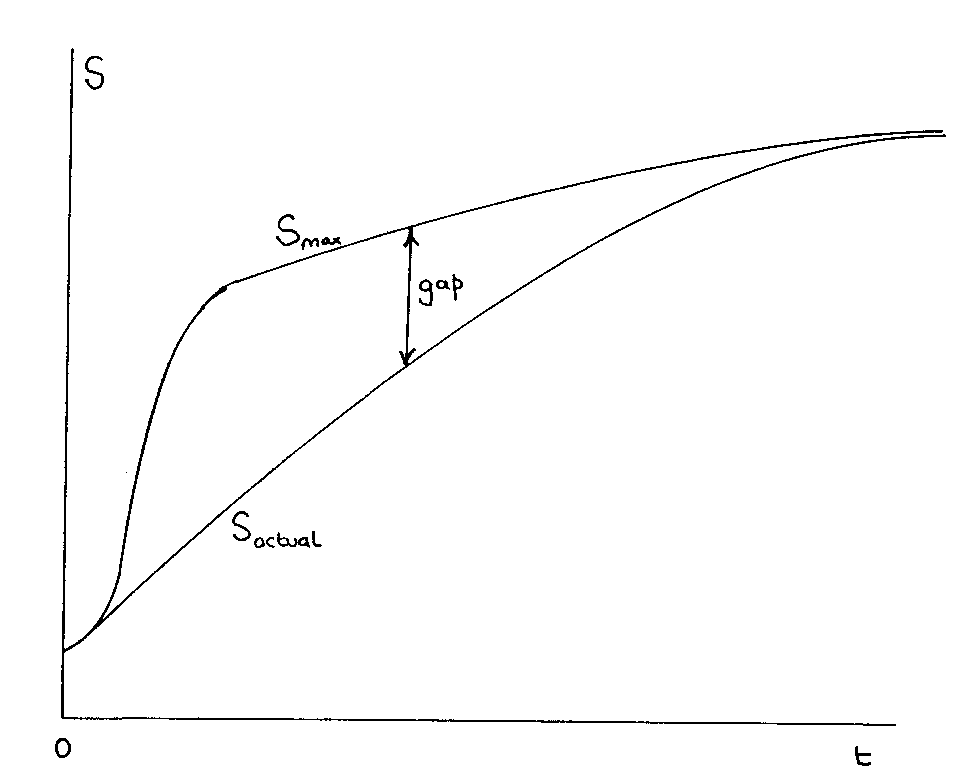 It is important to realise that the crucial effect of the expansion was in the early
universe - hence the sudden widening of the gap early on. Today it seems likely
(though I haven't checked) that the gap is narrowing: the universe produces copious
quantities of entropy at a rate which I imagine is faster than the (now rather feeble)
expansion raises the maximum possible entropy. The actual entropy will presumably
asymptote toward the maximum possible entropy in the very far future.
It is important to realise that the crucial effect of the expansion was in the early
universe - hence the sudden widening of the gap early on. Today it seems likely
(though I haven't checked) that the gap is narrowing: the universe produces copious
quantities of entropy at a rate which I imagine is faster than the (now rather feeble)
expansion raises the maximum possible entropy. The actual entropy will presumably
asymptote toward the maximum possible entropy in the very far future.
Physical Origins of Time Asymmetry, p.33
It's surprising that Davies suggests (though he hasn't checked!) that the entropy gap will close, leading to the 19th-century Kelvin-Helmholtz "
heat death of the universe." David Layzer had no such pessimism.
Davies suggests the closing of the "
entropy gap" will make the universe a
closed thermodynamic system!
Although before
life was created, there were no "agents" with "purposes" or "goals" and no "conscious minds," there were
values and
meaningful information structures. There were just no living beings in the
abiotic universe to see and appreciate that meaning.
The objective
value in the universe before the existence of life was the vast creation of negative entropy and
free energy, most critically their flow from the Sun to the Earth (as Schrödinger told us) to support the evolution and development of life in our
biosphere.
Normal |
Teacher |
Scholar