|
Philosophers
Mortimer Adler Rogers Albritton Alexander of Aphrodisias Samuel Alexander William Alston Anaximander G.E.M.Anscombe Anselm Louise Antony Thomas Aquinas Aristotle David Armstrong Harald Atmanspacher Robert Audi Augustine J.L.Austin A.J.Ayer Alexander Bain Mark Balaguer Jeffrey Barrett William Barrett William Belsham Henri Bergson George Berkeley Isaiah Berlin Richard J. Bernstein Bernard Berofsky Robert Bishop Max Black Susanne Bobzien Emil du Bois-Reymond Hilary Bok Laurence BonJour George Boole Émile Boutroux Daniel Boyd F.H.Bradley C.D.Broad Michael Burke Jeremy Butterfield Lawrence Cahoone C.A.Campbell Joseph Keim Campbell Rudolf Carnap Carneades Nancy Cartwright Gregg Caruso Ernst Cassirer David Chalmers Roderick Chisholm Chrysippus Cicero Tom Clark Randolph Clarke Samuel Clarke Anthony Collins Antonella Corradini Diodorus Cronus Jonathan Dancy Donald Davidson Mario De Caro Democritus Daniel Dennett Jacques Derrida René Descartes Richard Double Fred Dretske John Earman Laura Waddell Ekstrom Epictetus Epicurus Austin Farrer Herbert Feigl Arthur Fine John Martin Fischer Frederic Fitch Owen Flanagan Luciano Floridi Philippa Foot Alfred Fouilleé Harry Frankfurt Richard L. Franklin Bas van Fraassen Michael Frede Gottlob Frege Peter Geach Edmund Gettier Carl Ginet Alvin Goldman Gorgias Nicholas St. John Green H.Paul Grice Ian Hacking Ishtiyaque Haji Stuart Hampshire W.F.R.Hardie Sam Harris William Hasker R.M.Hare Georg W.F. Hegel Martin Heidegger Heraclitus R.E.Hobart Thomas Hobbes David Hodgson Shadsworth Hodgson Baron d'Holbach Ted Honderich Pamela Huby David Hume Ferenc Huoranszki Frank Jackson William James Lord Kames Robert Kane Immanuel Kant Tomis Kapitan Walter Kaufmann Jaegwon Kim William King Hilary Kornblith Christine Korsgaard Saul Kripke Thomas Kuhn Andrea Lavazza James Ladyman Christoph Lehner Keith Lehrer Gottfried Leibniz Jules Lequyer Leucippus Michael Levin Joseph Levine George Henry Lewes C.I.Lewis David Lewis Peter Lipton C. Lloyd Morgan John Locke Michael Lockwood Arthur O. Lovejoy E. Jonathan Lowe John R. Lucas Lucretius Alasdair MacIntyre Ruth Barcan Marcus Tim Maudlin James Martineau Nicholas Maxwell Storrs McCall Hugh McCann Colin McGinn Michael McKenna Brian McLaughlin John McTaggart Paul E. Meehl Uwe Meixner Alfred Mele Trenton Merricks John Stuart Mill Dickinson Miller G.E.Moore Ernest Nagel Thomas Nagel Otto Neurath Friedrich Nietzsche John Norton P.H.Nowell-Smith Robert Nozick William of Ockham Timothy O'Connor Parmenides David F. Pears Charles Sanders Peirce Derk Pereboom Steven Pinker U.T.Place Plato Karl Popper Porphyry Huw Price H.A.Prichard Protagoras Hilary Putnam Willard van Orman Quine Frank Ramsey Ayn Rand Michael Rea Thomas Reid Charles Renouvier Nicholas Rescher C.W.Rietdijk Richard Rorty Josiah Royce Bertrand Russell Paul Russell Gilbert Ryle Jean-Paul Sartre Kenneth Sayre T.M.Scanlon Moritz Schlick John Duns Scotus Arthur Schopenhauer John Searle Wilfrid Sellars David Shiang Alan Sidelle Ted Sider Henry Sidgwick Walter Sinnott-Armstrong Peter Slezak J.J.C.Smart Saul Smilansky Michael Smith Baruch Spinoza L. Susan Stebbing Isabelle Stengers George F. Stout Galen Strawson Peter Strawson Eleonore Stump Francisco Suárez Richard Taylor Kevin Timpe Mark Twain Peter Unger Peter van Inwagen Manuel Vargas John Venn Kadri Vihvelin Voltaire G.H. von Wright David Foster Wallace R. Jay Wallace W.G.Ward Ted Warfield Roy Weatherford C.F. von Weizsäcker William Whewell Alfred North Whitehead David Widerker David Wiggins Bernard Williams Timothy Williamson Ludwig Wittgenstein Susan Wolf Scientists David Albert Michael Arbib Walter Baade Bernard Baars Jeffrey Bada Leslie Ballentine Marcello Barbieri Gregory Bateson Horace Barlow John S. Bell Mara Beller Charles Bennett Ludwig von Bertalanffy Susan Blackmore Margaret Boden David Bohm Niels Bohr Ludwig Boltzmann Emile Borel Max Born Satyendra Nath Bose Walther Bothe Jean Bricmont Hans Briegel Leon Brillouin Stephen Brush Henry Thomas Buckle S. H. Burbury Melvin Calvin Donald Campbell Sadi Carnot Anthony Cashmore Eric Chaisson Gregory Chaitin Jean-Pierre Changeux Rudolf Clausius Arthur Holly Compton John Conway Simon Conway-Morris Jerry Coyne John Cramer Francis Crick E. P. Culverwell Antonio Damasio Olivier Darrigol Charles Darwin Richard Dawkins Terrence Deacon Lüder Deecke Richard Dedekind Louis de Broglie Stanislas Dehaene Max Delbrück Abraham de Moivre Bernard d'Espagnat Paul Dirac Hans Driesch John Dupré John Eccles Arthur Stanley Eddington Gerald Edelman Paul Ehrenfest Manfred Eigen Albert Einstein George F. R. Ellis Hugh Everett, III Franz Exner Richard Feynman R. A. Fisher David Foster Joseph Fourier Philipp Frank Steven Frautschi Edward Fredkin Augustin-Jean Fresnel Benjamin Gal-Or Howard Gardner Lila Gatlin Michael Gazzaniga Nicholas Georgescu-Roegen GianCarlo Ghirardi J. Willard Gibbs James J. Gibson Nicolas Gisin Paul Glimcher Thomas Gold A. O. Gomes Brian Goodwin Joshua Greene Dirk ter Haar Jacques Hadamard Mark Hadley Patrick Haggard J. B. S. Haldane Stuart Hameroff Augustin Hamon Sam Harris Ralph Hartley Hyman Hartman Jeff Hawkins John-Dylan Haynes Donald Hebb Martin Heisenberg Werner Heisenberg Grete Hermann John Herschel Basil Hiley Art Hobson Jesper Hoffmeyer Don Howard John H. Jackson William Stanley Jevons Roman Jakobson E. T. Jaynes Pascual Jordan Eric Kandel Ruth E. Kastner Stuart Kauffman Martin J. Klein William R. Klemm Christof Koch Simon Kochen Hans Kornhuber Stephen Kosslyn Daniel Koshland Ladislav Kovàč Leopold Kronecker Rolf Landauer Alfred Landé Pierre-Simon Laplace Karl Lashley David Layzer Joseph LeDoux Gerald Lettvin Gilbert Lewis Benjamin Libet David Lindley Seth Lloyd Werner Loewenstein Hendrik Lorentz Josef Loschmidt Alfred Lotka Ernst Mach Donald MacKay Henry Margenau Owen Maroney David Marr Humberto Maturana James Clerk Maxwell Ernst Mayr John McCarthy Warren McCulloch N. David Mermin George Miller Stanley Miller Ulrich Mohrhoff Jacques Monod Vernon Mountcastle Emmy Noether Donald Norman Travis Norsen Alexander Oparin Abraham Pais Howard Pattee Wolfgang Pauli Massimo Pauri Wilder Penfield Roger Penrose Steven Pinker Colin Pittendrigh Walter Pitts Max Planck Susan Pockett Henri Poincaré Daniel Pollen Ilya Prigogine Hans Primas Zenon Pylyshyn Henry Quastler Adolphe Quételet Pasco Rakic Nicolas Rashevsky Lord Rayleigh Frederick Reif Jürgen Renn Giacomo Rizzolati A.A. Roback Emil Roduner Juan Roederer Jerome Rothstein David Ruelle David Rumelhart Robert Sapolsky Tilman Sauer Ferdinand de Saussure Jürgen Schmidhuber Erwin Schrödinger Aaron Schurger Sebastian Seung Thomas Sebeok Franco Selleri Claude Shannon Charles Sherrington Abner Shimony Herbert Simon Dean Keith Simonton Edmund Sinnott B. F. Skinner Lee Smolin Ray Solomonoff Roger Sperry John Stachel Henry Stapp Tom Stonier Antoine Suarez Leo Szilard Max Tegmark Teilhard de Chardin Libb Thims William Thomson (Kelvin) Richard Tolman Giulio Tononi Peter Tse Alan Turing C. S. Unnikrishnan Nico van Kampen Francisco Varela Vlatko Vedral Vladimir Vernadsky Mikhail Volkenstein Heinz von Foerster Richard von Mises John von Neumann Jakob von Uexküll C. H. Waddington James D. Watson John B. Watson Daniel Wegner Steven Weinberg Paul A. Weiss Herman Weyl John Wheeler Jeffrey Wicken Wilhelm Wien Norbert Wiener Eugene Wigner E. O. Wilson Günther Witzany Stephen Wolfram H. Dieter Zeh Semir Zeki Ernst Zermelo Wojciech Zurek Konrad Zuse Fritz Zwicky Presentations Biosemiotics Free Will Mental Causation James Symposium |
Paul Dirac
Paul (P. A. M.) Dirac formulated the most elegant version of the mathematical principles of quantum mechanics after reading the proof copy of Werner Heisenberg's paper on the new "matrix mechanics."
A few months after the completion of matrix mechanics by Heisenberg’s mentor Max Born and Born’s assistant Pascual Jordan, Erwin Schrödinger developed his "wave mechanics." Dirac and Schrödinger independently showed the new wave mechanics was mathematically and physically equivalent to the Heisenberg picture, despite the extraordinary differences between the two quantum theories.
Almost two decades after Albert Einstein had said
It is therefore my opinion that the next stage in the development of theoretical physics will bring us a theory of light that can be understood as a kind of fusion of the wave and emission theories of light,Dirac's transformation theory gave us that "fusion" between waves and particles. Dirac combined the matrix and wave formulations using abstract symbolic methods from classical mechanics called Poisson brackets and canonical transformations. In his textbook The Principles of Quantum Mechanics, Paul Dirac introduced the new concepts of superposition of quantum states, the projection postulate, the axiom of measurement, and indeterminacy using simple examples with polarized photons. Dirac's examples suggest a very simple and inexpensive experiment that we call the Dirac 3-polarizers experiment to demonstrate the notions of quantum states, the preparation of quantum systems in states with known properties, the superposition of states, the measurement of various properties, the transformation or representation of a state vector in another basis set of vectors, and the infamous "collapse" or "reduction" of the wave function and the resulting indeterministic projection into one of the proper basis states. In their Copenhagen interpretation of quantum mechanics, Niels Bohr and Heisenberg said that the results of quantum measurements must be expressible in classical concepts because it is the language that humans can understand. By contrast, Dirac argued that the non-intuitive concepts of quantum mechanics, though impossible to understand in terms of classical concepts, could be mastered through long familiarity with them. The new theories, if one looks apart from their mathematical setting, are built up from physical concepts which cannot be explained in terms of things previously known to the student, which cannot even be explained adequately in words at all. Like the fundamental concepts (e.g. proximity, identity) which every one must learn on his arrival into the world, the newer concepts of physics can be mastered only by long familiarity with their properties and uses.
A Photon Interferes Only With Itself
In 1930, Dirac famously described a photon as interfering only with itself.
Consider a beam of light to be split into two components of equal intensity, which are made to interfere. According to the old corpuscular theory we would say that each of the two components contains an equal number of photons and we should then require that a photon in one component could interfere with one in the other. Under certain conditions they would have to annihilate one another, and under others to produce four photons. This contradicts the idea of photons being discrete particles and is, besides, in disagreement with the conservation of energy, which should hold for each process in detail and not be merely statistically true. The answer that quantum mechanics gives to the difficulty is that one should consider each photon to go partly into each of the two components, in the way allowed by the idea of the superposition of states. Each photon then interferes only with itself. Interference between two different photons can never occur. The solution of Maxwell’s equations that forms the wave picture of the phenomenon represents one of the photons and not the whole assembly of photons.In his later editions Dirac made the explanation more clear...
The scientist who "realized that the connection between light waves and photons must be of a statistical character" was of course Einstein.
Some time before the discovery of quantum mechanics people realized that the connexion between light waves and photons must be of a statistical character. What they did not clearly realize, however, was that the wave function gives information about the probability of one photon being in a particular place and not the probable number of photons in that place. The importance of the distinction can be made clear in the following way. Suppose we have a beam of light consisting of a large number of photons split up into two components of equal intensity. On the assumption that the intensity of a beam is connected with the probable number of photons in it, we should have half the total number of photons going into each component. If the two components are now made to interfere, we should require a photon in one component to be able to interfere with one in the other. Sometimes these two photons would have to annihilate one another and other times they would have to produce four photons. This would contradict the conservation of energy. The new theory, which connects the wave function with probabilities for one photon, gets over the difficulty by making each photon go partly into each of the two components. Each photon then interferes only with itself. Interference between two different photons never occurs.Regarding Dirac's claim that the wave function gives us "information about the probability of one photon being in a particular place and not the probable number of photons in that place," we should note that Einstein, and Born years later, strongly held both to be true. And we can give the reason. Dirac's quantum mechanics associates the quantum wave function with possibilities and a quantum particle with actualization of a possibility. Evaluating the Schrödinger equation lets us calculate the probabilities for each possibility, to an extraordinary degree of accuracy. Although the calculation involves abstract complex quantities and the motion through space of immaterial information about those possibilities, the result is both understandable (if non-intuitive because never experienced in our macroscopic world) and it is visualizable. We solve the Schrödinger equation given the boundary conditions to get the wave function. The boundary conditions are different when either one or two slits are open. So the probabilities of finding particles at the back screen are different, producing different interference fringes. These probabilities tell us where particles will be found, whichever slit the particles come through. Conservation laws (for energy, mass, charge, etc.) suggest that a particle comes through a single slit. It cannot divide into two photons or two electrons, or two buckyballs, despite Dirac's "manner of speaking." The resulting interference is described on the two-slit experiment page... Remembering that the double-slit interference appears even if only one particle at a time is incident on the two slits, we see why many say that the particle interferes with itself. But it is the wave function alone that is interfering with itself. Whichever slit the particle goes through, it is the probability amplitude ψ, whose squared modulus |ψ|2 gives us the probability of finding a particle somewhere, the interference pattern. It is what it is because the two slits are open. 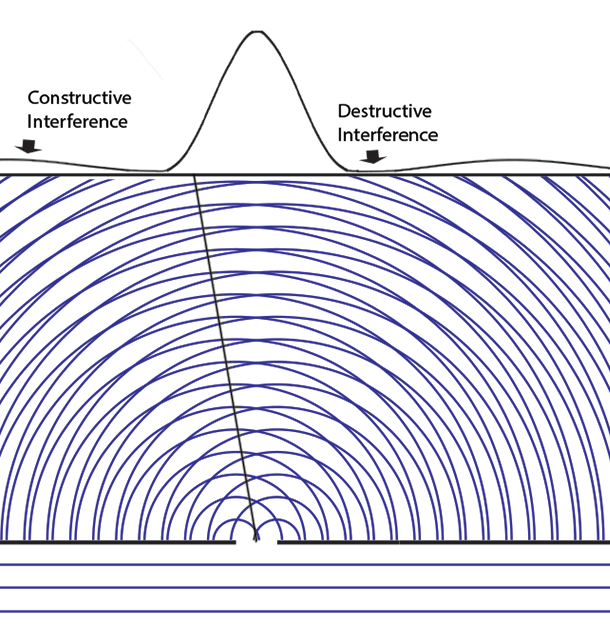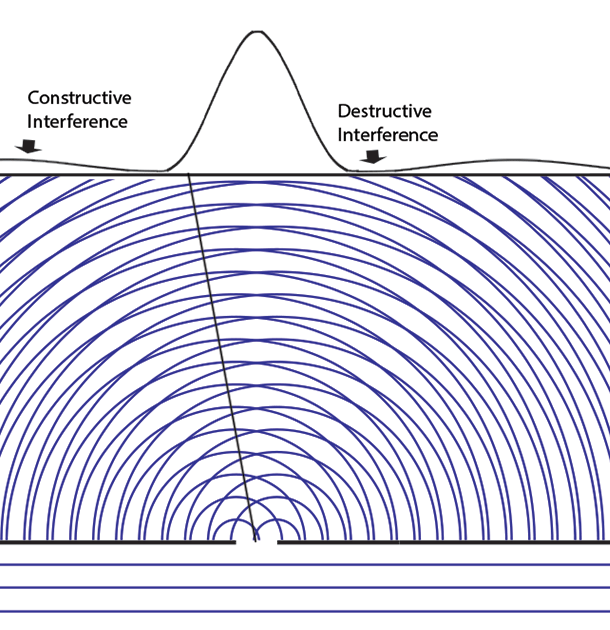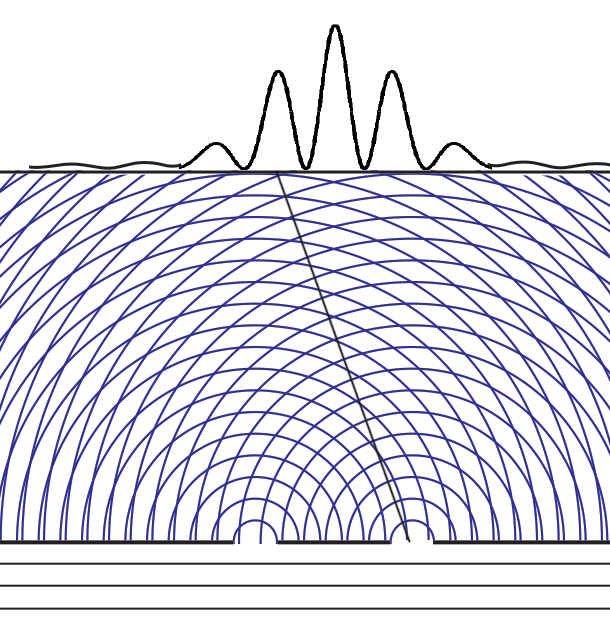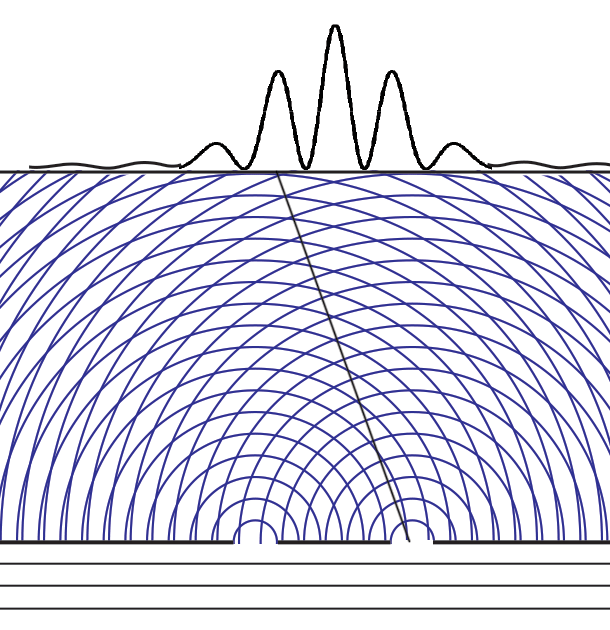
When you hear or read that electrons are both waves and particles, think "either-or" -
first a wave of possibilities, then an actual particle.
The Lagrangian in Quantum Mechanics
In 1932 Dirac wrote a short article, The Lagrangian in Quantum Mechanics, which became the basis for Richard Feynman's 1942 Princeton Ph.D thesis under the direction of John Wheeler. The article, published in the somewhat obscure journal Physikalisches Zeitschrift der Sowjetunion, was called to Feynman's attention in 1941 by a physicist emigrating from Nazi Germany, Herbert Jehle.
Feynman's thesis was titled "The Principle of Least Action in Quantum Mechanics." Following a section II called "Least Action in Classical Mechanics," Feynman's section III was called "Least Action in Quantum Mechanics," in which Section III.1 was called "The Lagrangian in Quantum Mechanics," the same title as Dirac's paper.
Feynman's thesis did not refer to Dirac's paper but to the new sections added to Dirac's classic text, "The Principles of Quantum Mechanics, in the 1935 and all later editions as "The Action Principle."
References
The Fundamental Equations of Quantum Mechanics, 1925
On the Theory of Quantum Mechanics, 1926
Relativity Quantum Mechanics with an Application to Compton Scattering, 1926
The Physical Interpretation of the Quantum Dynamics, 1927
The Quantum Theory of the Emission and Absorption of Radiation, 1927
From the Preface to The Principles of Quantum Mechanics, First Edition, 1930
Chapter 1 of The Principles of Quantum Mechanics, First Edition, 1930
The Lagrangian in Quantum Mechanics, 1933
On the Analogy Between Quantum and Classical Mechanics, 1945
Chapter 1 of The Principles of Quantum Mechanics, Fourth Edition, 1956
For Teachers
For Scholars
|